https://doi.org/10.1016/j.fct.2023.114157
T. Ruth a, J. Daniel b, A. Konig a, R. Trittler c, M. Garcia-Kaufer a
a Institute for Infection Prevention and Control, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Breisacher Strasse 115b, 79106, Freiburg, Germany
b Institute for Environmental Research, RWTH Aachen University, Worringerweg 1, 52074, Aachen, Germany
c Department of Pharmacy, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Hugstetter Strasse 55, 79106, Freiburg, Germany
A B S T R A C T
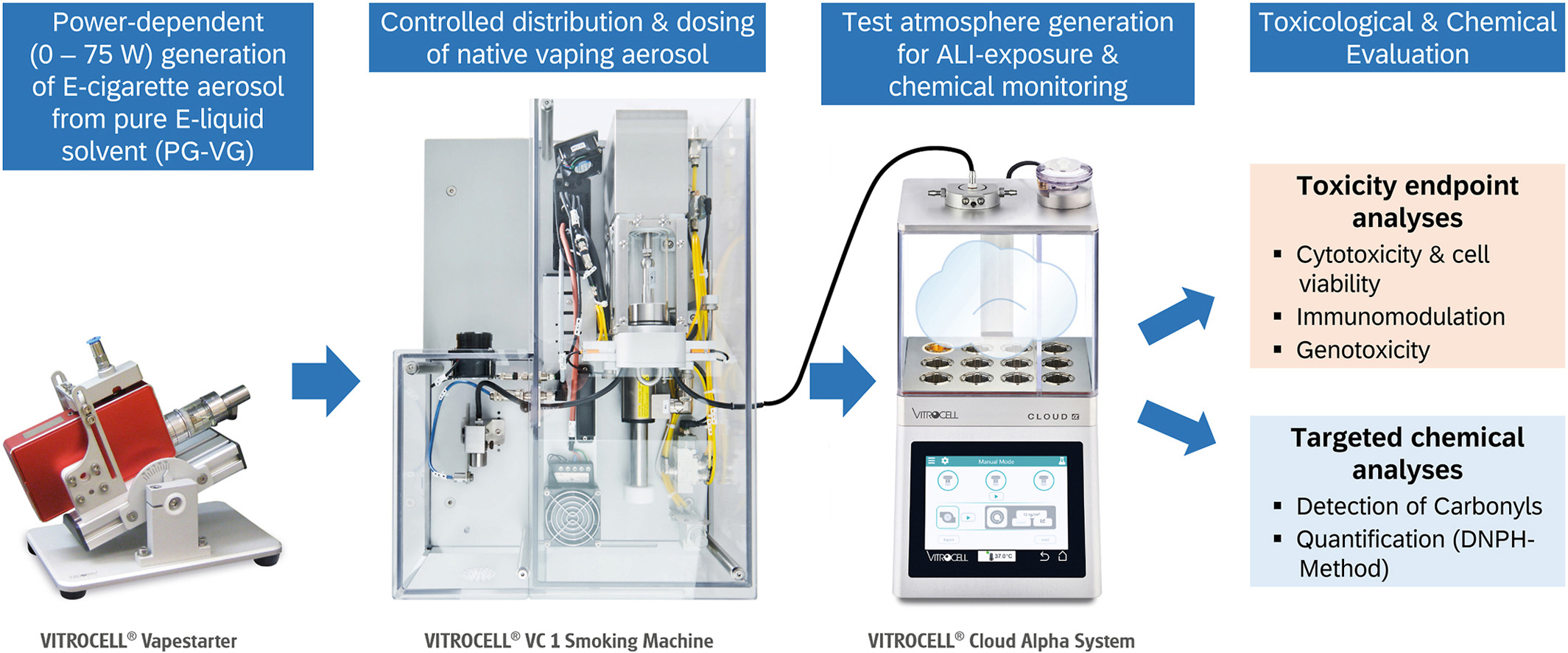
The increasing use of electronic nicotine delivery systems (ENDS), also known as e-cigarettes, has raised serious public health concerns, particularly regarding certain vaping product additives. The solvent carrier liquid, which consists of a mixture of propylene glycol (PG) and vegetable glycerine (VG), representing the main constituents of e-liquid formulations, have in contrast received little attention in health evaluations due to their apparent harmlessness when ingested; however, they can develop into potential lung hazards when heated, aerosolised and inhaled from ENDS with a user-defined heating profile. To assess the acute toxicity of the respirable aerosol, an effect-based in vitro testing strategy was applied in dependence of the heating power settings in ENDS. Human alveolar epithelial cells (A549) were exposed to vaping aerosol at the air–liquid interface (ALI), flanked by targeted chemical analyses of reactive carbonyl species. An exploratory, semi-automated in vitro exposure system provided evidence of a positive connection between vaporisation temperature and aerosol toxicity. Thermochemical transformation of the solvent leads to the formation of both cytotoxic and genotoxic substances that may disrupt lung homeostasis. Toxicity is therefore not limited to the presence of additives, as most harmful volatiles originate from the solvent itself, ultimately related to the device power output.